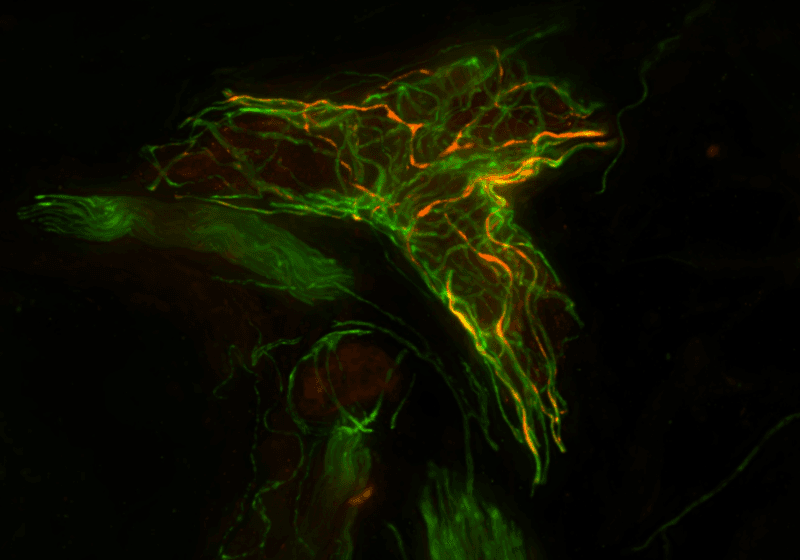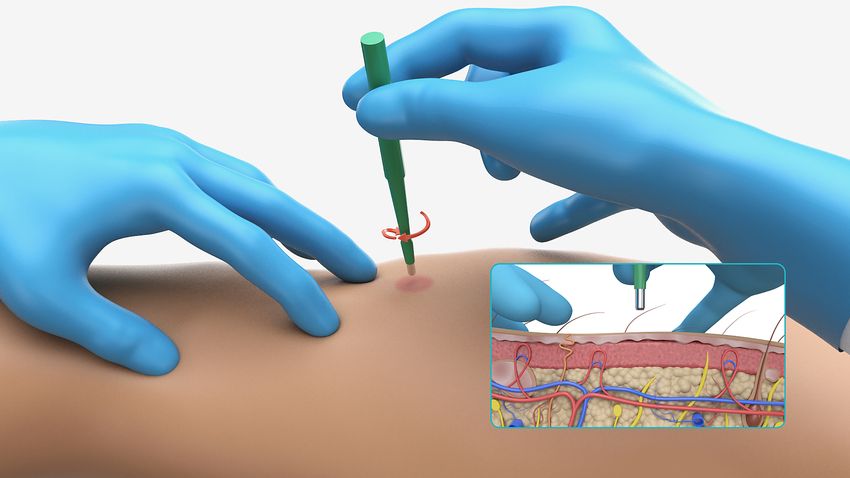

Assessing pathological synuclein in simple skin biopsies can take the place of more invasive diagnostic procedures. Diagnosing neurodegenerative conditions called synucleinopathies, particularly in early disease stages, has long been difficult due to overlapping clinical symptoms combined with the inaccessibility of the brain. Symptom-based guidelines and brain imaging tests often fall short, which has led scientists to develop biomarker-driven tools that can identify diseases earlier and more accurately.

Christopher Gibbons, MD, MMSc
Chief Scientific Officer and Co-founder
CND Life Sciences
In this Innovation Spotlight, Christopher Gibbons, the chief scientific officer and co-founder of CND Life Sciences, highlights a promising innovation called the Syn-One Test, which uses skin biopsies to detect pathological alpha-synuclein. This approach is reshaping how researchers study neurodegenerative disease progression and how clinicians diagnose these challenging-to-spot disorders.
What are synucleinopathies and how have they been historically diagnosed and studied?
Synucleinopathies are a group of neurodegenerative diseases characterized by the pathological accumulation of phosphorylated alpha-synuclein protein aggregates in the nervous system, most often within neurons or glial cells. Diseases known as synucleinopathies include Parkinson’s disease (PD), multiple system atrophy (MSA), and dementia with Lewy bodies (DLB).
Historically, definitive diagnosis of a specific synucleinopathy has relied on autopsy to examine brain and spinal cord tissue to detect phosphorylated alpha-synuclein. While recent clinical guidelines help clinicians diagnose synucleinopathies based on clusters of “red-flag” symptoms, accuracy is about 90 percent for late-stage disease but drops to 70 percent in early stages, highlighting the need for better diagnostic tools.1
This need has led to the development of techniques such as dopamine transporter imaging (DaTscan), which can help assess damage to the dopaminergic neurons in the substantia nigra. While useful, detecting a loss of these dopaminergic neurons is not specific to any one neurodegenerative disease, and this approach is expensive, time consuming, and requires very specialized infrastructure. Negotiating coverage for testing with insurers, limited access in rural settings, and potential contraindications with commonly used medicines all create challenges in the widespread use of DaTscan.
All of this leads to the current need for more sensitive and accessible diagnostic tools for synucleinopathies.
Why is early diagnosis so important for these disorders?
Early detection matters because these diseases progress differently despite very similar clinical presentations. For instance, PD and MSA can be difficult to differentiate in early stages of the disease. However, PD often unfolds over 20 years, while MSA progresses rapidly, with a median survival of just 3-7 years. For patients and families, knowing the specific diagnosis has a tremendous impact on life planning.
Accurate early diagnosis also informs safer care. DLB can mimic Alzheimer’s disease but often involves hallucinations and agitation. Antipsychotics, commonly used for Alzheimer’s disease, can cause severe, sometimes fatal reactions in patients with DLB. Early diagnosis can help avoid this risk.
A clear negative result is equally useful—ruling out PD, for example, may reveal treatable causes such as drug-induced tremors.
Finally, future disease-modifying therapies will only work if they are administered before significant neuron loss occurs. Stopping disease early is the goal.
What can biomarker assessment do for drug development and treatment of these diseases?
The hope with any biomarker is that it will ultimately allow us to more accurately diagnose disease in both early and late stages. Doing so can help us understand the natural history and underlying pathology of these conditions and home in on disease-specific drug targets. A biomarker supported diagnosis of these conditions in the early stages can also help ensure that clinical trials effectively target therapies to the right patient population at the right time.
That is the hope, and we are making progress towards that goal. There are two major biomarker classes in synucleinopathies that show promise. The first is imaging biomarkers. MRIs and nuclear medicine scanning technologies are used to assess structural patterns or proteins in the brain. As mentioned earlier, this approach can have limited accessibility, sensitivity, and specificity. At present, there are no imaging biomarkers in clinical use to detect synuclein proteins. Another biomarker approach is to examine the proteins causing these diseases. Within this class, there are two primary approaches. One is a technique called seed amplification where spinal fluid is collected and the biomarker signal, if present, is amplified to detectable levels. The second approach looks at existing protein levels located in skin neurons, which are accessed via skin biopsies.
The latter has significant potential, and not just because patients generally prefer skin biopsy over a spinal tap. Seed amplification technology makes it difficult to truly quantitate protein levels, which can be problematic if, for example, you’re measuring the success of therapeutic interventions based on changes in protein levels. The more quantitative a biomarker assessment, the better it is for drug developers to help guide their decision making.
How can skin biopsies be used for synucleinopathy diagnosis, and what benefit does this strategy provide?
Skin biopsies can work for synucleinopathies largely because the central nervous system and the peripheral nervous system are connected in a way that allows proteins to traffic between them. Alpha-synuclein is one of those proteins; it spreads both from the central to the peripheral nervous system and vice versa. This means that the protein can be widespread throughout the peripheral nervous system, including in the axons of dermal neurons.
A simple skin biopsy can then be used to assess pathological synuclein accumulation in peripheral neurons, and skin biopsies are far more accessible compared to brain or spinal fluid. Additionally, as noted earlier, this approach is quantitative unlike spinal fluid studies that rely on amplification to detect proteins.

Assessing pathological synuclein in simple skin biopsies can take the place of more invasive diagnostic procedures.
CND Life Sciences
How does the Syn-One Test work, and how can clinicians use this diagnostic to improve patient care?
The Syn-One Test uses three skin biopsies—one from the neck and two from the leg—to capture disease-specific patterns of alpha-synuclein deposition. Tissue samples are fixed, frozen, cut into thin sections, and stained for protein gene product 9.5 to detect neurons and for the phosphorylated, pathological form of alpha-synuclein, which is what matters diagnostically.
This test helps resolve diagnostic uncertainty. For example, if a patient’s tremor could be due to a medication side effect or PD, Syn-One can detect whether pathological synuclein is present, guiding the diagnosis and treatment plan.
What are some of the success stories coming out of trials using the Syn-One Test?
We have multiple longitudinal NIH trials that look at prodromal disease states. In one study, we recruit patients with REM sleep behavior disorder (RBD), a known prodromal synucleinopathy condition where patients act out their dreams. Over 15 years, 85-90 percent of patients with RBD will go on to develop PD or another synucleinopathy.
We already know that the Syn-One Test can detect phosphorylated alpha-synuclein in patients with clinically established synucleinopathies with a sensitivity and specificity of 95 percent, demonstrating its ability to accurately identify these conditions.2
Through these trials, we have also now shown that we’re able to detect with the Syn-One Test this misfolded protein in 75 percent of people with idiopathic RBD.3,4 One of our major remaining questions is whether skin biopsy results can predict who is going to develop a synucleinopathy and which one they’re likely to be diagnosed with. We think the results of these studies will be very exciting.
There are other trials in which drug developers are using the Syn-One Test to improve patient homogeneity, ensuring that only patients with phosphorylated alpha-synuclein are included in their studies. Other companies are using the Syn-One Test to study the natural history of synuclein accumulation over time—here we have seen some early signs of success that we’re excited about.
What is on the horizon for the Syn-One Test?
We are excited about some recent developments in confocal imaging that allow us to do high-throughput imaging and digitization of skin biopsies. We’ve developed a software tool with very powerful machine learning algorithms to help quantify nerve fibers and protein detection, enabling us to analyze protein localization across the whole network of nerves in the biopsy and potentially measure changes over time. This could be a very powerful tool to support drug development and clinical trial development.
What are some of the challenges and future possibilities surrounding precision medicine in neurodegenerative disease care and research?
One of the big challenges with neurodegenerative diseases is that they can be very kaleidoscopic in their clinical presentation. There may be multiple overlapping conditions happening at once, such as Alzheimer’s disease where 30-40 percent of patients have co-pathology that is associated with worse outcomes. Personalized medicine will require us to improve our understanding of neurodegenerative processes generally, and specifically how the various drivers of pathology, such as synuclein aggregates, interact with one another to bring about disease. The more we can understand these diseases at a biomarker level, the better we can predict disease progression and develop precision medicine.
On the practical side, this is a challenging field to work in because it can take a long time to get adequate validation of disease predictions and interventions. There are successes happening and we are seeing an increase in laboratory derived tests that can help with disease diagnosis, but they still require quite a bit of validation. It will be a while before these are used regularly in clinical practice, but we’re beginning to entertain the idea that they soon will be.

Source link